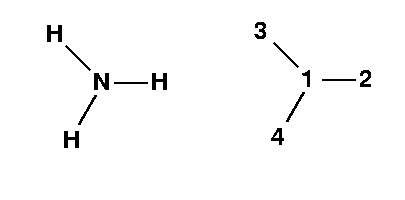
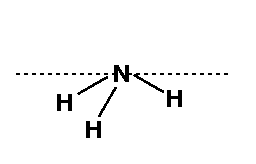


It is important to realize that the H atoms all lie 20° below the dashed line. There will be 1 dehedral angle in this molecule. It will be the 4-1-2-3 angle. Draw a Newman Projection.
(CH3)2CO :
The numbering of the atoms in acetone:
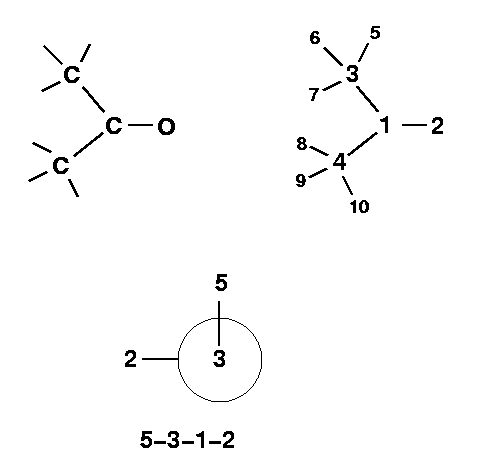
Also notice, I have included the Newman Projection of the 5-3-1-2 angle.
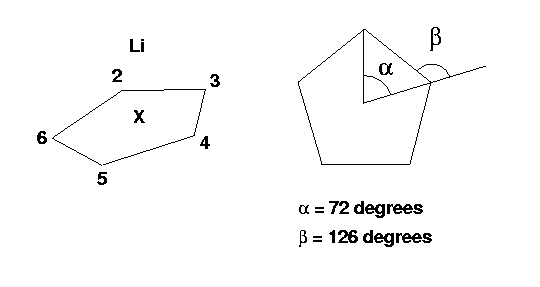
This is what the molecule looks like and rememeber the C5H5 moiety is planar in this case. The hydrogen atoms are omitted for clarity.