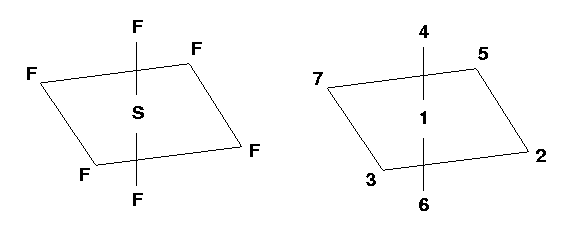

Atom 1 is the Sulfur atom and Atom 2 is the first Fluorine atom. Atom 2 is connected to Atom 1, which makes the first S-F bond.
Atom 3: Atom 3 is the second Fluorine atom. It is
"connected" to Atom 1 (bond length=R) and makes an angle of 90° with Atom 2.
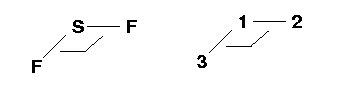
Atom 4: Atom 4 is the third Fluorine atom. It is
"connected" to Atom 1 (bond length=R) and makes an angle of 90° with Atom 2. It also
makes a dihedral angle (4-1-2-3) of 90°. Look down the 1-2 axis...
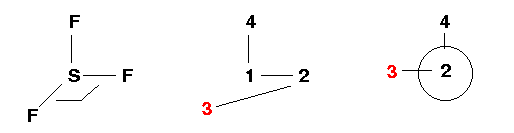
Now you may something like "2 is not bonded to 3". You would be absolutely correct too! However, in the Z-matrix formulation "2 is connected to 3" in the sense that they define a dihedral angle (4-1-2-3). This instance shows the strength of the Z-matrix.
Atom 5: Atom 5 is the fourth Fluorine atom. It is
"connected" to Atom 1 (bond length=R) and makes an angle of 90° with Atom 2. It also
makes a dihedral angle (5-1-2-3) of 180° with Atom 3.
Look down the 1-2 axis...
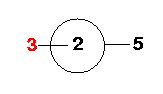
Atom 6: Atom 6 is the fifth Fluorine atom. It is
"connected" to Atom 1 (bond length=R) and makes an angle of 90° with Atom 2. It also
makes a dihedral angle (6-1-2-3) of -90° with Atom 3.
Look down the 1-2 axis...
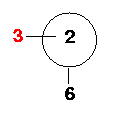
Atom 7: Atom 7 is the last Fluorine atom. It is
"connected" to Atom 1 (bond length=R) and makes an angle of 90°
with Atom 3. It also makes a dihedral angle (7-1-3-2) of 180° with
Atom 2. Look at the figure... Look down the
1-3 axis...
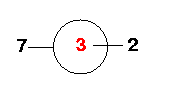
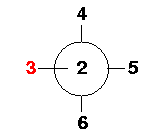
Here is a composite drawing of SF6 down the 1-2
axis which shows the placement of Atoms 2-6 with respect to
each other. Atom 2 should be viewed as coming out of the page
toward the reader. Atom 7 lies along the 1-2 axis and is
therefore hidden in this view.