


Next: A Phase-field Model
Up: Materials Science Modeling
Previous: Phase-Field Dendritic Growth
G. B. McFadden ACMD
W. J. Boettinger, Materials Science and Engineering Laboratory
A. A. Wheeler, University of Southampton
Solidification of a eutectic alloy from the melt or liquid phase
produces two composite solid phases, in contrast to the simpler case when
the liquid phase solidifies into a single solid phase. The two solid
phases are characterized by differing solute concentrations;
the equilibrium concentrations in each phase are given by the
phase diagram for the eutectic system, which gives the
relations between temperature and solute concentration for each of the
thermodynamic phases in the system. A number of different
geometrical arrangements of the two solid phases are possible, depending
on the alloy system and on the processing conditions. For example,
the solid phases can form in adjacent layers or lamellae, or one
phase can form rods that are embedded in the other phase.
Both grow continuously from the melt.
Since a large number of important binary alloy systems have
eutectic phase diagrams, such systems have been studied extensively.
It is desirable to be able to predict the geometrical patterns of
growth and their associated length scales in order to better understand
and possibly control the processing of eutectic materials.
Phase-field methodologies for the solidification of single phase
solids for single component or pure materials were developed
in the early 1980's. In these models an auxiliary variable
or order parameter, known as the
phase field, is introduced in order to differentiate in a continuous
fashion between the liquid and solid phases in the system. In its simplest form,
these models include a thermodynamic description of the free energy of the system
as a function of the temperature and the phase field.
Phase-field models for the
solidification of two-component systems having
simple phase diagrams were developed in the last several years by the
above authors and by researchers elsewhere. These models feature
a free energy that depends on the solute concentration in addition
to the temperature and the phase field; the free energy is constructed in
such as way that the appropriate phase diagrams for the system are
recovered. More recently, we have
developed models for solidification of eutectic systems. The
most general model includes a formulation for the free energy of the
system in terms of the temperature and concentration of the system,
together with two order parameters rather than a single one.
One of the order parameters, 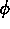 , is used to indicate whether the system
is in a solid or liquid phase, and the other order parameter,
, is used to indicate whether the system
is in a solid or liquid phase, and the other order parameter, 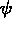 ,
is used to indicate which of the two possible solid phases is present.
With this free energy it is possible to recover the appropriate
eutectic phase diagram for the system.
,
is used to indicate which of the two possible solid phases is present.
With this free energy it is possible to recover the appropriate
eutectic phase diagram for the system.
The resulting phase-field model has been used to study the formation
of trijunctions in which both solid phases are in equilibrium with the
liquid phase. The angles formed by the inter-phase boundaries should
meet at specific angles that satisfy Young's law, representing
a balance of surface energies at the trijunction. An example is shown
in the accompanying figure, where a temperature gradient is imposed
in the vertical direction. The top figure shows the trijunction region,
consisting of the liquid phase above the two solid phases, labeled
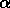 and
and 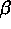 . Contour lines are shown for the solute field.
The middle figure shows contour lines for the phase field
. Contour lines are shown for the solute field.
The middle figure shows contour lines for the phase field 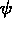 ,
which determines the solid-solid interface.
The lower figure shows contour lines for the phase field
,
which determines the solid-solid interface.
The lower figure shows contour lines for the phase field 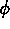 , which
determines the solid-liquid interfaces.
, which
determines the solid-liquid interfaces.
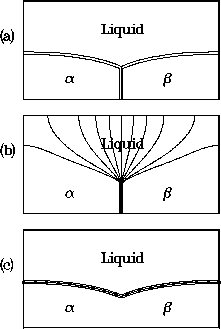
Figure 6: Phase-field computation for a eutectic trijunction
situated in a temperature gradient, showing
contours of (a) the concentration field, (b) the phase field 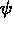 ,
and (c) the phase field
,
and (c) the phase field 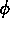 .
.



Next: A Phase-field Model
Up: Materials Science Modeling
Previous: Phase-Field Dendritic Growth
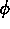 , is used to indicate whether the system
is in a solid or liquid phase, and the other order parameter,
, is used to indicate whether the system
is in a solid or liquid phase, and the other order parameter, 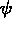 ,
is used to indicate which of the two possible solid phases is present.
With this free energy it is possible to recover the appropriate
eutectic phase diagram for the system.
,
is used to indicate which of the two possible solid phases is present.
With this free energy it is possible to recover the appropriate
eutectic phase diagram for the system.
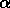 and
and 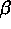 . Contour lines are shown for the solute field.
The middle figure shows contour lines for the phase field
. Contour lines are shown for the solute field.
The middle figure shows contour lines for the phase field 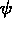 ,
which determines the solid-solid interface.
The lower figure shows contour lines for the phase field
,
which determines the solid-solid interface.
The lower figure shows contour lines for the phase field 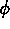 , which
determines the solid-liquid interfaces.
, which
determines the solid-liquid interfaces.

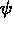 ,
and (c) the phase field
,
and (c) the phase field 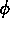 .
.